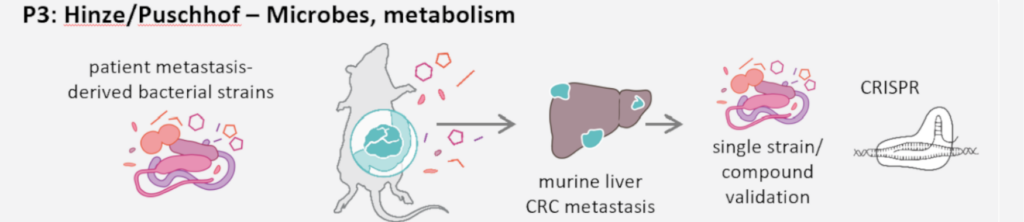
The functional implications of the intra-metastasis microbiome are also largely unknown. For this reason, L. Hinze and J. Puschhof hypothesise that the interplay of microbiota-derived factors and metabolic changes influences niche dependency, metastasis phenotypes and thereby therapy response in CRC metastases. Previously, they identified ribosomal proteins of the large and small subunits as drivers of niche independence. In P3 they aim to validate these hits using targeted in vitro and in vivo CRISPR approaches in the presence or absence of microbes and bacterial metabolites to identify modulators of CRC metastases. Furthermore, they will utilise patient biopsies with co-derived bacterial strains and organoids to identify individual bacterial strains and metabolites that shape metastasis cell behaviour and therapy responsiveness. To elucidate the mechanistic underpinnings of these interactions, they will combine innovative organoid-bacteria co-cultures and assays developed in the Puschhof group with the expertise on metabolic assays, CRISPR screening and drug screening in vitro and in vivo present in the Hinze group.